Gatalin® XL meets all the relevant regulatory requirements of a bioequivalent medicine (UK reference product Reminyl® (galantamine) XL 8mg, 16mg and 24mg prolonged-release capsules [Takeda]).1 Three bioequivalence clinical trials were performed comparing Reminyl XL 8mg prolonged-release capsules with Gatalin XL 8mg prolonged-release capsules. Two single dose studies were performed under fed and fasted conditions and a steady state study was performed under fasted conditions. The graphs are shown below.
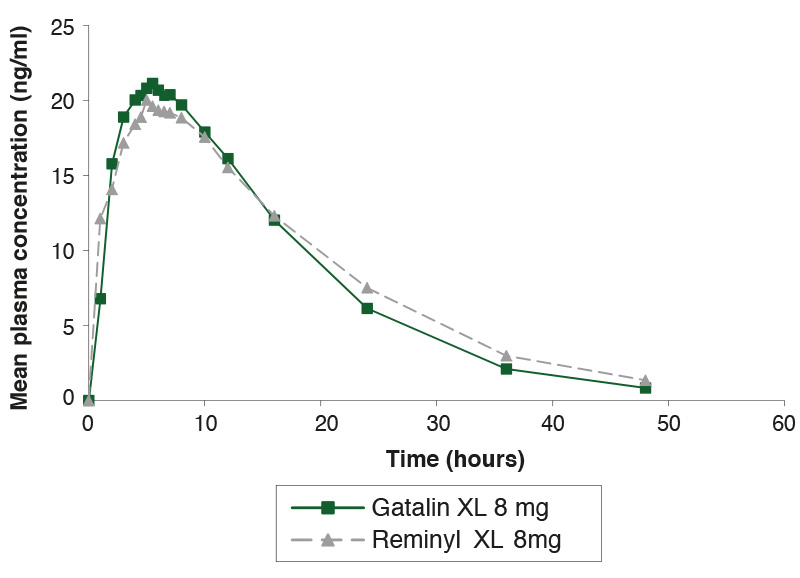
Single-dose study comparing Gatalin XL 8mg and Reminyl XL 8mg under fasting conditions
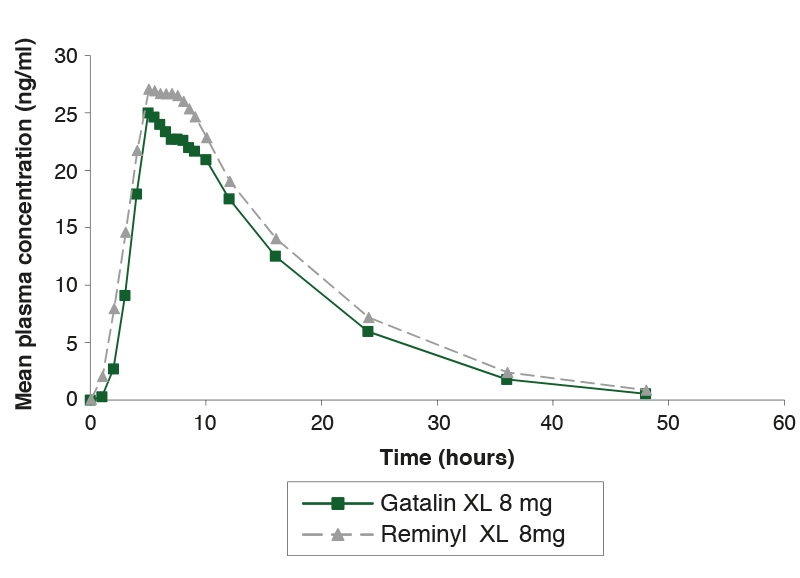
Single-dose study comparing Gatalin XL 8mg and Reminyl XL 8mg under fed conditions
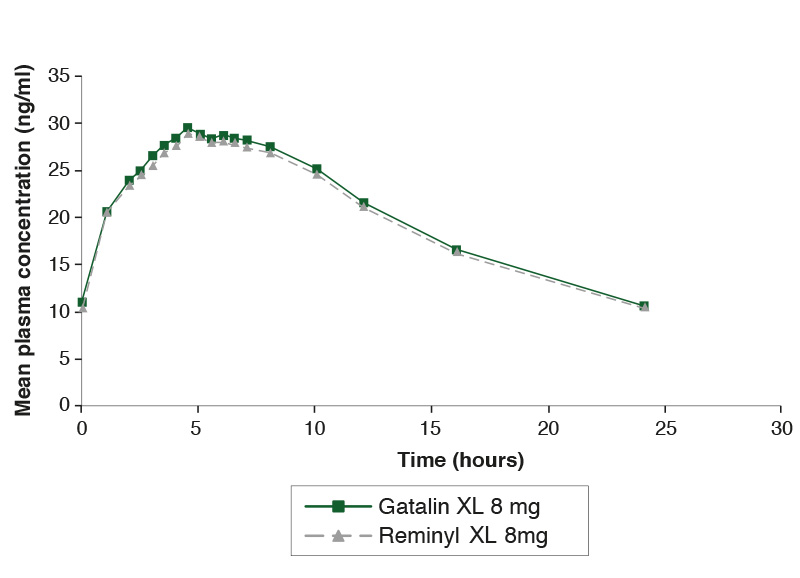
Steady-state study comparing Gatalin XL 8mg and Reminyl XL 8mg under fasting conditions
References: 1) Data on file. 1010067149 v 6.0 September 2023.
For more information about Gatalin XL, please see the abbreviated prescribing information.















